Understanding the Specialized Nature of Medical Device Molding
The medical manufacturing industry demands exceptional precision, quality, and compliance in every component produced. Medical mold production represents a highly specialized segment of injection molding that goes far beyond standard industrial applications. While conventional molds serve various commercial and industrial purposes, medical molds must meet stringent requirements to ensure patient safety and regulatory compliance.
Medical molds are specifically engineered to produce components for healthcare applications, from surgical instruments to diagnostic devices. These molds require extraordinary attention to detail, superior material selection, and adherence to strict manufacturing protocols that distinguish them from their standard counterparts.
Critical Design and Engineering Considerations
Enhanced Precision Requirements
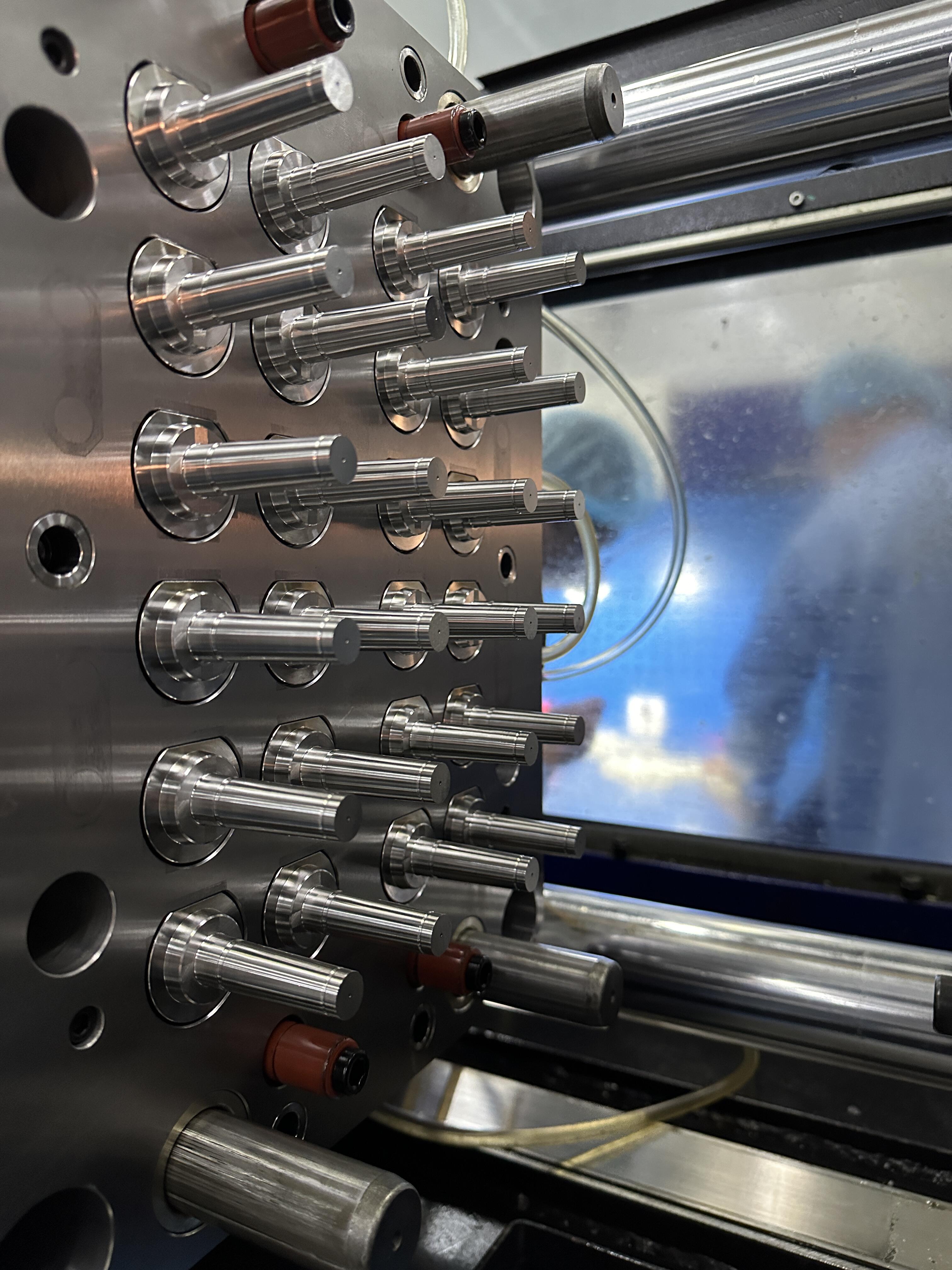
Medical mold design demands unprecedented levels of precision. Unlike standard molds that might tolerate minor variations, medical molds must consistently produce parts with exact specifications, often down to micron-level tolerances. This precision is crucial for components that will be used in critical medical procedures or implanted in the human body.
Engineers must account for material shrinkage, cooling patterns, and gate locations with extreme accuracy. The design process involves sophisticated computer modeling and simulation to ensure perfect part reproduction every time. This level of precision often requires specialized CAD/CAM software and advanced manufacturing techniques not typically used in standard mold production.
Material Selection and Compatibility
Medical molds must be constructed from materials that meet specific medical-grade requirements. While standard molds might use conventional tool steels, medical molds often require specialized stainless steels or other materials that resist corrosion and can withstand aggressive sterilization processes. The mold material must also be compatible with the medical-grade resins used in production.
Furthermore, these materials need to maintain their integrity through numerous production cycles while preventing any possibility of contamination or degradation that could affect the final medical components. This often means using higher-grade materials and more sophisticated surface treatments than those found in standard molds.
Regulatory Compliance and Documentation
Quality Management Systems
Medical mold manufacturing operates under strict quality management systems that far exceed typical industrial standards. These systems must comply with ISO 13485 requirements specifically designed for medical device manufacturing. Every aspect of the mold's design, production, and validation must be documented and traceable.
Quality control measures include detailed documentation of material certifications, production parameters, and validation testing. This level of documentation and control is rarely necessary for standard molds but is absolutely essential in medical applications to ensure consistent quality and regulatory compliance.
Validation and Testing Protocols
The validation process for medical molds is substantially more rigorous than for standard molds. Each mold must undergo extensive testing and validation protocols to ensure it consistently produces parts that meet all specified requirements. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) processes.
Testing protocols often involve multiple production runs under various conditions to verify consistent performance. Statistical analysis of produced parts helps ensure the mold maintains tight tolerances throughout its operational life. These validation requirements add significant complexity and cost to medical mold development compared to standard molds.
Specialized Production Environment
Cleanroom Manufacturing Requirements
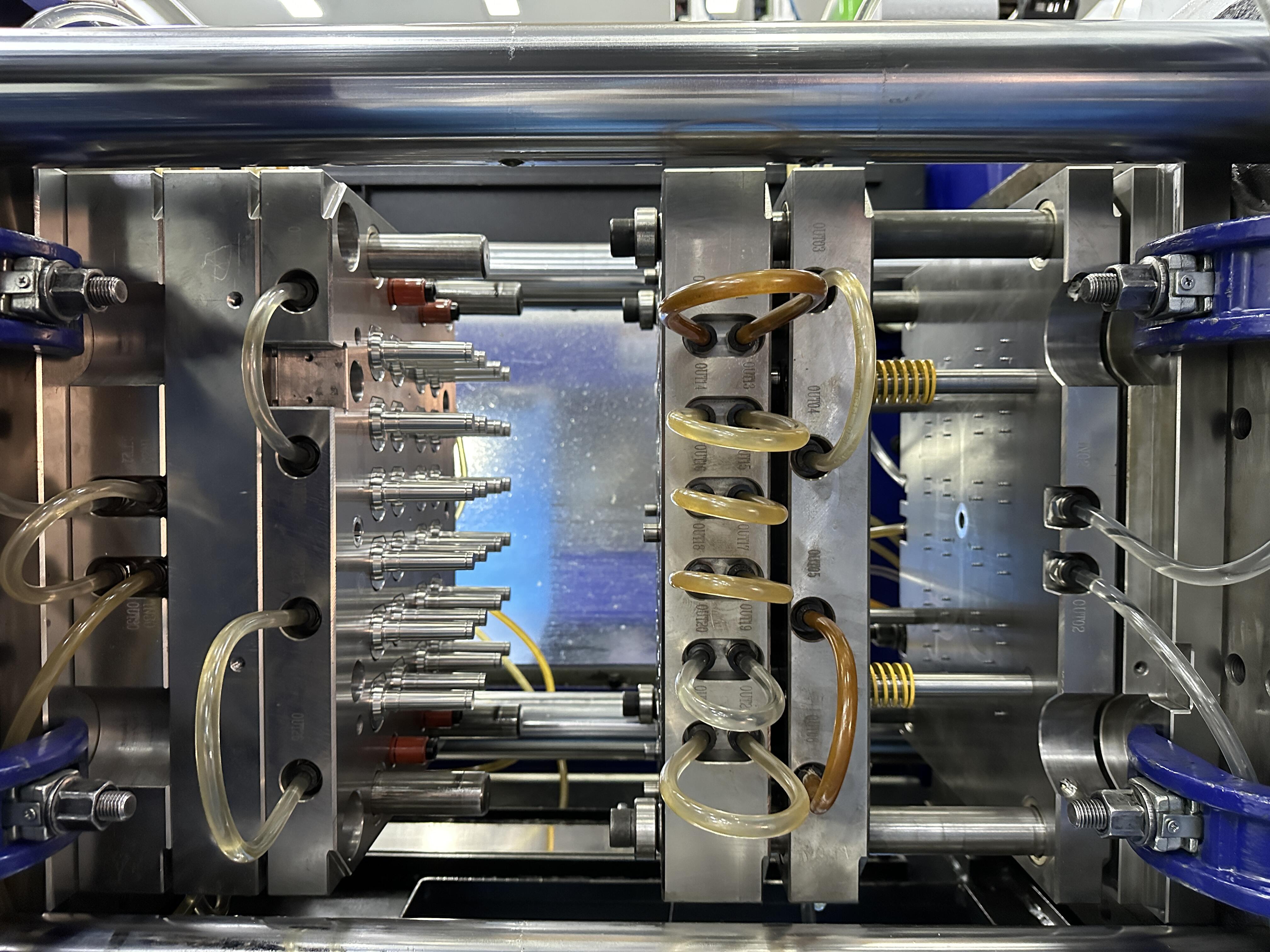
Medical molds typically operate in controlled cleanroom environments, unlike standard molds that function in conventional manufacturing settings. These cleanrooms must maintain specific air quality, temperature, and humidity levels to prevent contamination and ensure consistent production quality.
The cleanroom requirement affects not only the production environment but also the design of the mold itself. Medical molds must be engineered to minimize particle generation and facilitate easy cleaning and maintenance within the cleanroom setting. This often requires special design features and surface treatments not found in standard molds.
Sterilization Compatibility
Medical molds must be designed to withstand regular sterilization processes, which can include steam autoclaving, gamma radiation, or chemical treatments. This requirement influences material selection and design features in ways that standard molds never need to consider. The mold components must maintain their dimensional stability and performance characteristics even after repeated sterilization cycles.
The ability to thoroughly clean and sterilize medical molds between production runs is crucial for preventing cross-contamination and maintaining product quality. This often requires special design features such as polished surfaces, minimal parting lines, and accessible cleaning areas that aren't typically necessary in standard molds.
Frequently Asked Questions
How long does medical mold development typically take compared to standard molds?
Medical mold development generally requires 2-3 times longer than standard mold development due to extensive design reviews, validation requirements, and regulatory compliance processes. While a standard mold might be completed in 8-12 weeks, a medical mold could take 20-30 weeks or longer.
What makes medical molds more expensive than standard molds?
Medical molds typically cost significantly more due to several factors: higher-grade materials, more precise machining requirements, extensive validation testing, documentation requirements, and the need for specialized design features. The total cost can be 2-5 times higher than comparable standard molds.
How often do medical molds need to be validated?
Medical molds require initial validation before production begins and periodic revalidation throughout their lifecycle. Revalidation is typically performed annually or after any significant maintenance, repair, or process change. Some applications may require more frequent validation based on regulatory requirements or risk assessment.